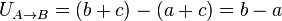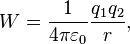Overview
Potential energy exists when a force acts upon an object that tends to restore it to a lower energy configuration. This force is often called a restoring force. For example, when a spring is stretched to the left, it exerts a force to the right so as to return to its original, unstretched position. Similarly, when a mass is lifted up, the force of gravity will act so as to bring it back down. The action of stretching the spring or lifting the mass requires energy to perform. The energy that went into lifting up the mass is stored in its position in the gravitational field, while similarly, the energy it took to stretch the spring is stored in the metal. According to the law of conservation of energy, energy cannot be created or destroyed; hence this energy cannot disappear. Instead, it is stored as potential energy. If the spring is released or the mass is dropped, this stored energy will be converted into kinetic energy by the restoring force, which is elasticity in the case of the spring, and gravity in the case of the mass. Think of a roller coaster. When the coaster climbs a hill it has potential energy. At the very top of the hill is its maximum potential energy. When the car speeds down the hill potential energy turns into kinetic. Kinetic energy is greatest at the bottom.The more formal definition is that potential energy is the energy difference between the energy of an object in a given position and its energy at a reference position.
There are various types of potential energy, each associated with a particular type of force. More specifically, every conservative force gives rise to potential energy. For example, the work of an elastic force is called elastic potential energy; work of the gravitational force is called gravitational potential energy; work of the Coulomb force is called electric potential energy; work of the strong nuclear force or weak nuclear force acting on the baryon charge is called nuclear potential energy; work of intermolecular forces is called intermolecular potential energy. Chemical potential energy, such as the energy stored in fossil fuels, is the work of the Coulomb force during rearrangement of mutual positions of electrons and nuclei in atoms and molecules. Thermal energy usually has two components: the kinetic energy of random motions of particles and the potential energy of their mutual positions.
As a general rule, the work done by a conservative force F will be



 ,
, pairs of two bodies, the potential energy of the system of those two bodies.
pairs of two bodies, the potential energy of the system of those two bodies.