Chemical potential energy
Main article: Chemical energy
Chemical potential energy is a form of potential energy related to the structural arrangement of atoms or molecules. This arrangement may be the result of chemical bonds within a molecule or otherwise. Chemical energy of a chemical substance can be transformed to other forms of energy by a chemical reaction. As an example, when a fuel is burned the chemical energy is converted to heat, same is the case with digestion of food metabolized in a biological organism. Green plants transform solar energy to chemical energy through the process known as photosynthesis, and electrical energy can be converted to chemical energy through electrochemical reactions.The similar term chemical potential is used to indicate the potential of a substance to undergo a change of configuration, be it in the form of a chemical reaction, spatial transport, particle exchange with a reservoir, etc.
Electric potential energy
Main article: Electric potential energy
An object can have potential energy by virtue of its electric charge and several forces related to their presence. There are two main types of this kind of potential energy: electrostatic potential energy, electrodynamic potential energy (also sometimes called magnetic potential energy). Plasma formed inside a gas filled sphere.
Electrostatic potential energy
In case the electric charge of an object can be assumed to be at rest, it has potential energy due to its position relative to other charged objects.The electrostatic potential energy is the energy of an electrically charged particle (at rest) in an electric field. It is defined as the work that must be done to move it from an infinite distance away to its present location, in the absence of any non-electrical forces on the object. This energy is non-zero if there is another electrically charged object nearby.
The simplest example is the case of two point-like objects A1 and A2 with electrical charges q1 and q2. The work W required to move A1 from an infinite distance to a distance r away from A2 is given by:
This equation is obtained by integrating the Coulomb force between the limits of infinity and r.
A related quantity called electric potential (commonly denoted with a V for voltage) is equal to the electric potential energy per unit charge.

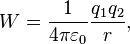
No comments:
Post a Comment